Pancreatic glucagon is produced in islet α cells following posttranslational processing of proglucagon by specific prohormone convertase enzymes. The relative islet a-cell mass may be reduced in diabetic subjects Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988 Dec;9(4):151-9. but limited data is available on this topic. Although the processing of proglucagon in the α cell remains under active investigation, current evidence supports an important role for PC2 in this process, however whether PC2 alone directly cleaves proglucagon to glucagon remains unclear. For example, see Proglucagon is processed to glucagon by prohormone convertase PC2 in alpha TC1-6 cells Proc Natl Acad Sci U S A 1994 Apr 12;91(8):3242-6; Processing of mouse proglucagon by recombinant prohormone convertase 1 and immunopurified prohormone convertase 2 in vitro J Biol Chem 1995 Apr 28;270(17):10136-46; Evidence for redundancy in propeptide/prohormone convertase activities in processing proglucagon: an antisense study Mol Endocrinol. 1996 Apr;10(4):331-41
A major component of the phenotype exhibited by the PC2 knockout mouse, namely mild hypoglycemia and marked alpha cell hyperplasia, appears attributable to defective levels of circulating glucagon and reduced Gcgr signal transduction, as glucagon replacement by osmotic mini-pump corrected hypoglycemia and produced a significant decrease in the number of hyperplastic alpha cells. The islet remodeling was detectable by 11 days, and after 25 days, PC2-/- islets resembled wild type islets. Apoptosis of islet α cells appears to contribute to the remodeling process, implying an important role for a threshold of circulating glucagon in the regulation of both islet α cell proliferation and survival. See Glucagon Replacement via Micro-Osmotic Pump Corrects Hypoglycemia and a-Cell Hyperplasia in Prohormone Convertase 2 Knockout Mice. Diabetes. 2002 Feb; 51(2): 398-405
Plasticity of the islet α cell phenotype and interconversion of islet α- and b-cells is a topic of active interest. Bramswig and colleagues used epigenetic methylation markers to compare potential transcriptional activation states in DNA corresponding to thousands of genes isolated from sorted purified human islet α and b cells and exocrine cells. Remarkably, a-cells contained a greater proportion of bivalent histone modifications and treatment of cultured human islets with a histone methyltransferase inhibitors induced co-localization of α-and b-cell markers in the same islet cell. These findings support the concept of epigenetic regulation of cellular plasticity in α-cells. Epigenomic plasticity enables human pancreatic α to β cell reprogramming J Clin Invest. 2013 Feb 22. doi:pii: 66514. 10.1172/JCI66514
Longuet et al. assessed the GCGR-dependent signals and tissues responsible for induction of islet α cell hyperplasia in both Gcgr-/- mice and in mice with liver-specific inactivation of the Gcgr. Inactivation of the hepatic Gcgr recapitulated the phenotype of whole body Gcgr-/- mice, including marked islet α cell hyperplasia, improved oral and intraperitoneal glucose tolerance, and reduced fasting glucose. Remarkably transplantation of Gcgr+/+ islets into either Gcgr-/- recipients, or GcgrHep-/- recipients, resulted in proliferation of islet α cells in transplanted +/+ islets underneath the kidney capsule. These findings imply that the liver responds to interruption of the glucagon receptor pathway by initiating one or more signals that promote robust and rapid islet α cell proliferation independent of the normal islet localization and pancreatic location. Liver-Specific Disruption of the Murine Glucagon Receptor Produces α-Cell Hyperplasia: Evidence for a Circulating α-Cell Growth Factor Diabetes published ahead of print November 16, 2012, doi:10.2337/db11-1605
Remarkably few α cells are required to maintain euglycemia and normal plasma glucagon levels. Thorel and colleagues used a genetic approach employing expression of diphtheria toxin in murine α cells to ablate 98% of α cell mass. Remarkably, mice maintained normal levels of glucose, insulin, and glucagon despite massive deficiency of α cells. Although pancreatic glucagon content dropped substantially, the remnant α cells were capable of increasing glucagon synthesis and secretion, yet did not initially proliferate in response to diminished α-cell mass, however by 6 months after DT-induced ablation, α-cell mass had doubled. Furthermore the liver exhibited acutely enhanced glucagon sensitivity (enhanced glucose mobilization after IP glucagon administration). Lineage tracing experiments showed that beta cells do not undergo reprogramming to become α-cells following α-cell ablation. Normal Glucagon Signaling and {beta}-Cell Function After Near-Total {alpha}-Cell Ablation in Adult Mice Diabetes. 2011 Nov;60(11):2872-82.
Does the α cell developmentally regulate or contribute to the formation of islet β cells and vice versa?
Studies in the developing murine pancreas using embryonic pancreas cultures in vitro demonstrate that reduction of pancreatic proglucagon expression markedly diminishes the formation of insulin+ b-cells in the early embryonic pancreas around E11. This effect was attributable to glucagon, and not GLP-1. These intriguing observations need to be reconciled with apparently normal β cell development reported in the glucagon receptor-/- mouse. See Glucagon is required for early insulin-positive differentiation in the developing mouse pancreas. Diabetes. 2002 Nov;51(11):3229-36
Experimental evidence from destruction of murine b-cells suggests that regeneration of b-cell mass under certain circumstances may proceed through an intermediary step involving islet a-cells. Administration of diphtheria toxin to mice engineered to express the DT receptor on b-cells results in destruction of b-cells, followed by slow but progressive regeneration of b-cell mass, without detectable changes in proliferation of pre-existing b-cells. Co-expression of glucagon and insulin was detected in many islet cells during the regeneration process. Genetic tagging of a-cells prior to b-cell destruction revealed that pre-existing monohormonal a-cells then started to co-express insulin after DT-mediated b-cell destruction. Furthermore, destruction of a-cells using a similar DT approach markedly prevented the regeneration of b-cells in this model. See Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss Nature. 2010 Apr 4;. [Epub ahead of print]
A related mouse expressing the Diptheria toxin (DT) receptor under the control of the proglucagon promoter (1.6 kb fragment) was used to examine the consequences of severe a-cell depletion in vivo. Massive (98 percent) a-cell necrosis/apoptosis was detected within 2 days of DT administration, resulting in a 99% reduction in glucagon content. Remarkably, blood glucose was maintained in the normal range in the fasted or fed states despite massive a-cell depletion. Furthermore, the counter-regulatory response to insulin-induced hypoglycemia was also normal in these mice, likely due to unexpected preservation of the glucagon secretory capacity of the remaining a-cells. The sensitivity to exogenous glucagon was also modestly increased. Some limited a-cell regeneration was noted in older mice. The authors conclude that the mouse pancreatic a-cells contain sufficiently large glucagon reserves to preserve levels of circulating glucagon despite massive a-cell depletion. Normal Glucagon Signaling and β-Cell Function After Near-Total α-Cell Ablation in Adult Mice Diabetes published ahead of print September 16, 2011, doi:10.2337/db11-0876
The transcription factor Arx appears to be downstream of ngn-3 and is essential for the formation of a-cells in the endocrine pancreas, as targeted deletion of Arx in mice results in hypoglycemia, dehydration, weakness, early neonatal lethality in association with a marked depletion of a-cells from the endocrine pancreas, with a corresponding increase in the numbers of b- and d-cells. See Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 2003 Oct 15;17(20):2591-603. A subsequent study used the Pdx1-CRE mouse to delete Arx specidfically from the pancreas. Pancreas-specific loss of Arx leads to a marked loss in numbers of a-cells and increased numbers of b, d and PP cells. Consistent with glucagon deficiency, glucose tolerance was markedly improved in Arx-deficient mice, that also accumulated fat and glycogen in the liver. Glucagon deficiency reduces hepatic glucose production and improves glucose tolerance in adult mice Mol Endocrinol. 2010 Aug;24(8):1605-14
a-cells may also be derived from human b-cells ex vivo. Spijker used a form of lineage tracing (lentiviral infection of human islet cultures) to assess formation of "new a-cells" in islets formed after dispersion of islets into single cells, followed by reaggregation. Insulin gene expression and the number of insulin immunopositive b-cells declined after several days, whereas the number of glucagon+ a-cells increased, independent of major differences in cell proliferation or apoptosis. After 14 days of islet aggregation and culture, up to 15% of cultured GFP+ islet cells were glucagon immunopositive. The glucagon+GFP+ population persisted even after transplantation of islet cultures under the kidney capsule of NOD/SCID mice. The interconversion of b-cells into a-cells in this model system was attenuated by knockdown of the transcription factor Arx. Conversion of mature human β-cells into glucagon-producing α-cells Diabetes. 2013 Apr 8.
Further evidence for the importance of Arx in mice and humans was revealed in Partial loss of pancreas endocrine and exocrine cells of human ARX-null mutation: consideration of pancreas differentiation Differentiation. 2010 Sep-Oct;80(2-3):118-22 with naturally ocurring Arx mutations. The transcription factor islet-1, previously shown to be an important regulator of islet proglucagon gene transcription, is also an important determinant of Arx expression in islet a-cells. Arx expression is reduced in islet-1-/- pancreas and transgenic over-expression of islet-1 in islets increases expression of Arx. The effect of islet-1 may be located through binding sites in the 3'-flanking region of the Arx gene Islet-1 regulates Arx transcription during pancreatic islet {alpha}-cell development. J Biol Chem. 2011 Mar 9. [Epub ahead of print]. Indeed, forcing the expression
Forced mis-expression of Arx in the embryonic pancreas or developing beta cells produces a marked loss of b-cells, increased numbers of a-cells and PP cells, apparently through conversion of b-cells to a- and PP cells, illustrating that b-cells can be "reprogrammed" to a-cells through instructions directed by Arx Embryonic endocrine pancreas and mature beta cells acquire alpha and PP cell phenotypes upon Arx misexpression J Clin Invest. 2007 Apr;117(4):961-70.
Dhawan and colleagues identified an important role for methylation as a critical epigenetic process controlling repression of Arx in b-cells, and in turn maintenance of the b-cell phenotype. Genetic deletion of DNA methyltransferase (Dnmt1) in murine b-cells using the Rip-CRE transgene produced no initial phenotype, however with increasing time (6-8 months of age), the mice developed loss of DNA methylation in b-cells; this occurs primarily as a consequence of newly formed replicating b-cells losing the activity of Dnmt1, leading to an increased number of islet a-cells, and glucose intolerance. Analysis of promoter methylation identified Arx as a candidate transcription factor differentially methylated in islet a vs b-cells. Clonal analysis of methylation patterns revealed 2 populations of b-cells in b-cell-specific Dnmt1-/- mice, one with normal b-cell methylation, and a second clonal population that was unmethylated at the Arx gene locus, resembling the pattern found in a-cells. Furthermore FACS purified b-cells from older mice exhibited increased Arx and MafB and reduced Pdx1 and Pax4 mRNA transcripts, consistent with an a-cell phenotype. MeCP2 was identified as a methyl binding protein contributing to the repression of Arx transcriptional axctivity in b-cells, and MeCP2 was found to associate with PRMT6 (protein arginine methyltransferase 6, a known H3R2 methyltransferase that represses transcription by antagonizing H3K4me3. Hence Dnmt1 controls b vs. a-cell fate by regulating methylation at the Arx locus leading to changes in the balance of transcriptional repressors vs. activators of Arx. Pancreatic β Cell Identity Is Maintained by DNA Methylation-Mediated Repression of Arx Dev Cell. 2011 Apr 19;20(4):419-29
The interest in understanding the developmental and cellular origin of pancreatic islet b-cells has fostered numerous studies that have greatly informed our understanding of α cell development. The widespread application of knockout technology to the study of islet transcription factor genes has provided important new insights into our understanding of islet α cell development. For an overview, see Pancreas: how to get there from the gut? Curr Opin Cell Biol. 1999 Dec;11(6):663-8 Original developmental studies detected a population of islet cells that co-expressed insulin and glucagon, implying a developmental lineage relationship between these 2 cell types, as in Development. 1993 Aug;118(4):1031-9. However ablation of either α or β cells using promoter-driven diphtheria toxin did not compromise development of the alternative cell lineage as shown in Ablation of islet endocrine cells by targeted expression of hormone- promoter- driven toxigenes. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12999-3003. Furthermore, Pedro Herrera confirmed these findings using an elegant combination of islet hormone promoter-driven Cre recombinase transgenes to activate and mark cell precursors expressing a reporter transgene. The results of these studies provide additional evidence for independent origin of islet α and β cells, and reaffirm a role for pdx-1 as an upstream regulator of α cell development as illustrated in Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127(11):2317-2322. This view is also supported by independent studies of islet development, which show that ngn-3 expression identifies a population of ngn3+/pdx1+ cells that independently give rise to separate islet α and β cell lineages (Diabetes 2000 49:163-176).
Pdx-1 is also a dominant regulator of the a versus the b-cell phenotype. The roles of brain4 and pdx-1 were examined in an elegant set of experiments employing conditional activation and repression of pdx-1 and brn4 expression in both a and b-cell lines. Over-expression of Pdx1 eliminated glucagon mRNA and protein in INSralphabeta cells. Induction of dominant-negative Pdx1 in INSralphabeta cells resulted in differentiation of insulin-producing beta-cells into glucagon-containing alpha-cells without altering brain4 expression. Loss of Pdx1 function alone in INSrbeta cells, which do not express endogenous brain-4 and glucagon, was also sufficient to abolish the expression of genes restricted to beta-cells and to cause alpha-cell differentiation. In contrast, induction of brain-4 in INSrbeta cells initiated detectable expression of glucagon but did not affect beta-cell-specific gene expression. See Pdx1 level defines pancreatic gene expression pattern and cell lineage differentiation. J Biol Chem. 2001 Jul 6;276(27):25279-86. and Suppression of Pdx-1 perturbs proinsulin processing, insulin secretion and GLP-1 signalling in INS-1 cells. Diabetologia. 2005 Apr;48(4):720-31.
Furthermore, reduction of pdx-1 expression in mouse b-cells using a conditional antisense approach resulted in an increased expression of glucagon in islets of these mice. See The Tet-On system in transgenic mice: inhibition of the mouse pdx-1 gene activity by antisense RNA expression in pancreatic beta-cells. J Mol Med. 2001 Jun;79(5-6):321-8.
A complementary approach tested the ability of ectopically expressed brn4 to induce glucagon gene expression in islet b-glucagon expressing alpha cell lineage, even in the context of the beta cell lineage. See Brn-4 Transcription Factor Expression Targeted to the Early Developing Mouse Pancreas Induces Ectopic Glucagon Gene Expression in Insulin-producing beta Cells. J Biol Chem. 2002 May 3;277(18):16028-32
Nevertheless, genetic inactivation of Brn-4 in the mouse does not produce a defect in islet a-cell development or levels of pancreatic glucagon, as shown in The role of Brn4/Pou3f4 and Pax6 in forming the pancreatic glucagon cell identity. Dev Biol. 2004 Apr 15;268(1):123-34.
An increasing number of genes have now been identified as important for α cell development through mouse knockout and transgenic approaches, including isl-1, pax-6, Nkx2.2, neurogenin-3, HNF-6, and N-CAM,
The pou homeodomain transcription factor brn-4 plays a role in the specification of islet α cell development at a precise time during islet cell development. Expression of brn-4 under the control of the pdx-1, but not the insulin promoter, induces the co-expression of glucagon and insulin in the beta cell lineage of transgenic murine islets in vivo. Similarly, brn-4 expression in transfected rat AR42J cells resulted in the induction of proglucagon gene expression in vitro. In contrast, expression of pax-6 using the same pdx-1 promoter did not induce ectopic glucagon gene expression in beta cells. See Brn-4 transcription factor expression targeted to the early developing mouse pancreas induces Ectopic glucagon gene expression in insulin-producing beta-cells. J Biol Chem. 2002 Feb 7
Does the islet α cell produce GLP-1 in the context of islet development, regeneration, or hyperglycemia?
Several studies support this possibility by demonstrating induction of PC1 expression in isolated cultured islet α cells. Although GLP-1 is not normally synthesized in physiologically relevant amounts in normal islet α cells, the amount of pancreatic GLP-1 may be increased in rats with STZ-induced diabetes. Pancreatic and α cell expression of PC1 is induced in rats given streptozotocin, leading to small but significant increases in the levels of bioactive GLP-1 in the rat pancreas. The islet α cell expression of both prohormone convertase enzymes PC1 and PC2 are upregulated in this model. Regulation of pancreatic PC1 and PC2 associated with increased glucagon-like peptide 1 in diabetic rats : J Clin Invest 2000 Apr 1;105(7):955-965. The physiological significance of these findings remains unknown, but may be related to an adaptive response to experimental islet injury.
A second study documents the developmental expression of proglucagon and PC1 in the embryonic mouse pancreas from E10.5 to E 15.5 Expression pattern of IAPP and prohormone convertase 1/3 reveals a distinctive set of endocrine cells in the embryonic pancreas. Mech Dev. 2002 Jul;115(1-2):171-176, raising the possibility that bioactive GLP-1 might be liberated from these cells with potential implications for b-cell growth and development. Similarly, rats treated with streptozotocin exhibit b-cell regeneration, increased pancreatic levels of GLP-1 and the antagonist exendin(9-39) diminished the b-cell regeneration observed after STZ administration Ontogeny of regeneration of beta-cells in the neonatal rat after treatment with streptozotocin. Endocrinology. 2006 May;147(5):2346-56. These studies do not prove that bioactive GLP-1 exerts a role during development or regeneration, but they certainly expand our concepts to include a potential for some islet α cells to produce GLP-1 in the correct developmental or experimental setting in the context of b-cell injury.
Consistent with a role for inflammation or disruption of islet integrity in the phenotypic switching of a subset of islet a-cells that produce exclusively glucagon to a-cells capable of expression PC1 and producing GLP-1, IL-6 has been shown to increase PC1 expression and GLP-1 production in rodent and human islet cells Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells Nat Med. 2011 Oct 30;17(11):1481-9. , and immunoneutralization of IL-6 by systemic injection of IL-6 antisera starting immediately after birth impaired a-cell formation, and reduced pancreatic Gcg gene expression and circulating levels of glucagon in the developing neonatal Wistar rat Role of endogenous IL-6 in the neonatal expansion and functionality of Wistar rat pancreatic alpha cells Diabetologia. 2013 Feb23
GLP-1 production from islet a-cells has also been observed following complete or partial reduction of glucagon receptor signaling. Mice with targeted disruption of the Gcgr gene, or rodents with reduced glucagon receptor expression develop islet a-cell hyperplasia and increased pancreatic and plasma GLP-1, as described in Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):1438-43 and in Hepatic and glucagon-like peptide-1–mediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors J. Clin. Invest. 113:1571-1581 (2004).
Marchetti et al evaluated the presence of active GLP-1 in cultured human islets from non-diabetic and diabetic donors. A few a-cells immunopositive for PC1/3 were detected by immunohistochemistry in sections from human pancreas and some a-cells were also immunopositive for GLP-1 immunoreactivity in about 3/4 of islets examined. Human pancreatic islets also secreted active GLP-1, as veriified by specific assays and Mass Spc analysis of tryptic digests from islet extracts. A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets Diabetologia. 2012 Sep 11.
Islet injury, high glucose levels, or TGR5 activation have been associated with enhanced PC1 expression and concomitant GLP-1 production in islets or aTC-1 cells Processing of proglucagon to GLP-1 in pancreatic {alpha}-cells: is this a paracrine mechanism enabling GLP-1 to act on {beta}-cells? J Endocrinol. 2011 Oct;211(1):99-106
The gerbil Psammomys obesus exhibits nutrient-sensitive b-cell failure and development of T2DM on a high energy Western diet. Hansen and colleagues demonstrated that levels of amidated and bioactive GLP-1 were increased (~2-fold) in pancreatic extracts from hyperglycemic high energy fed P. obesus animals; portal vein plasma concentrations of intact GLP-1 and intact GLP-1 released from cultured islets was also higher in hyperglycemic animals. Active GLP-1 release was also detected in human islet cultures and immunohistochemistry using antisera against PC1/2 revealed expression in diabetic a-cells from gerbils and perhaps in some human islets Upregulation of alpha cell glucagon-like peptide 1 (GLP-1) in Psammomys obesus-an adaptive response to hyperglycaemia? Diabetologia. 2011 Feb 25. [Epub ahead of print]
Liu and colleages demonstrated transient neonatal expression of the chemokine SDF-1 in murine b-cells, and SDF-1 expression in vitro was induced by injury with toxins, cellular stressors, and cytokines. SDF-1 itself further induced SDF-1 gene expression in b-cells through an autoregulatory feedback loop, through a signaling pathway involving Jak/STAT signaling. Alpha cells express the SDF-1 receptor (CXCR4) and SDF-1 activated AKT phsophorylation in a-cells in vitro; exogenous SDF-1 induced expression of prohormone convertase-1 and stimulated GLP-1 production from mouse and human islets cultured in vitro. Stromal cell-derived factor-1 (SDF-1)/chemokine (C-X-C motif) receptor 4 (CXCR4) axis activation induces intra-islet glucagon-like peptide-1 (GLP-1) production and enhances beta cell survival Diabetologia. 2011 May 13. [Epub ahead of print]
Genes and proteins in the islet a-cell
What do we understand about the molecular components of the a-cell? In contrast to the considerable amount of data available about the genes and proteins expressed in b-cells, there is limited information available about RNA transcripts and proteins expressed in a-cells. The transcription factor profiles of glucagon-producing cells have been described in Differentiation phenotypes of pancreatic islet beta- and alpha-cells are closely related with homeotic genes and a group of differentially expressed genes. Gene. 2004 Apr 28;331:53-63. Similar studies contrasting a and β cells have been described in Contrasting patterns of expression of transcription factors in pancreatic alpha and beta cells. Proc Natl Acad Sci U S A. 2003 Oct 28;100(22):12660-5. To determine whether profiling a-cell transcripts at the RNA level yields complementary or overlapping information relative to that obtained with proteomic approaches, Maziarz and colleagues have compared proteomic vs RNA profiles obtained in aTC cells. These studies have identified unique attributes of proteomic vs. Affymetrix expression profiling technologies for as described in Integrating global proteomic and genomic expression profiles generated from islet a cells:Opportunities and challenges to deriving reliable biological inferences. Mol Cell Proteomics. 2005 Mar 1;
AIMP1/p43 is a multifunctional cytokine that is also expressed in islet a-cells and secreted in response to hypoglycemia. Exogenous administration of AIMP1 induced glucagon secretion in rats and stimulates glucagon secretion from isolated a-TC9 cells in vitro. AIMP1-/- mice also exhibited reduced levels of plasma glucose, improved glucose tolerance, and reduced levels of plasma insulin and glucagon-See Hormonal activity of AIMP1/p43 for glucose homeostasis. Proc Natl Acad Sci U S A. 2006 Sep 25; [Epub ahead of print]
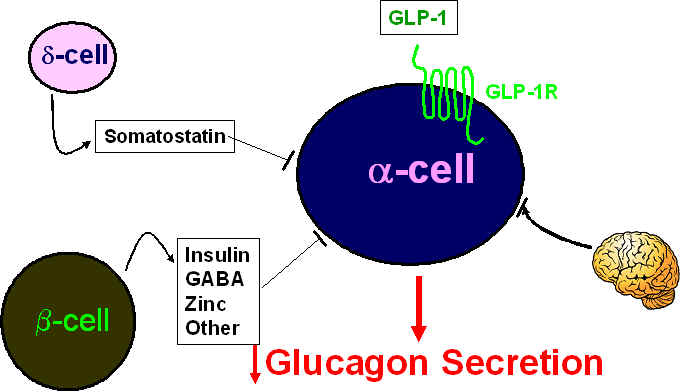
How does GLP-1 inhibit glucagon secretion from the islet a-cell? The precise mechanisms remain unclear but likely involve a combination of pathways including one or more b-cell-derived products, somatostatin from the d-cell, direct coupling of the a-cell GLP-1R (expressed in some but not all a-cells) to inhibition of secretion, and/or the involvement of neural signals as depicted below.