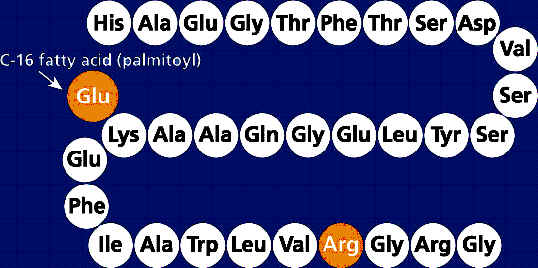
Arg(34)Lys(26)-(N- epsilon -(gamma-Glu(N-alpha-hexadecanoyl))-GLP-1(7-37)
 Liraglutide (Victoza) ,
a long-acting DPP-4-resistant GLP-1R
agonist, has been developed
for the treatment of type 2 diabetes, although exploratory studies of Liraglutide action in obese non-diabetic subjects are underway. Liraglutide was approved in the United States on January 25 2010 for the treatment of type 2 diabetes. Review the US Liraglutide Prescribing Information and the FDA Questions and Answers - Safety Requirements for Victoza (liraglutide) and Liraglutide Patient Prescribing Information and for an overview of all comprehensive US prescribing and patient safety information, see www.victoza.com
Liraglutide (Victoza) ,
a long-acting DPP-4-resistant GLP-1R
agonist, has been developed
for the treatment of type 2 diabetes, although exploratory studies of Liraglutide action in obese non-diabetic subjects are underway. Liraglutide was approved in the United States on January 25 2010 for the treatment of type 2 diabetes. Review the US Liraglutide Prescribing Information and the FDA Questions and Answers - Safety Requirements for Victoza (liraglutide) and Liraglutide Patient Prescribing Information and for an overview of all comprehensive US prescribing and patient safety information, see www.victoza.com
The FDA perspective on the Liraglutide approval was published in the NEJM on Feb 17 2010
Liraglutide was approved in Europe in the spring of 2009 and received marketing authorization on June 30 2009. The drug is indicated for patients failing to achieve optimal control on metformin alone, metformin+SU, or metformin+TZD therapy. There is insufficient information in regard to use of the drug in patients with liver disease or with moderete to severe renal impairment and the drug should not be used in pregnant or breast feeding women. The drug require storage in the fridge in between useds. See the complete Liraglutide EMEA (Victoza) prescribing information.
Liraglutide is largely resistant to the actions of DPP-4 via its amino acid addition and acylation, albumin binding, and its amino acid substitution. However like Similarly to GLP-1, liraglutide was cleaved in vitro by DPP-4 in the Ala8-Glu9 position of the N-terminal and degraded by NEP into several metabolites, including a GLP-1(9-37)-like metabolite in human subjects, as evident from human studies described in Metabolism and Excretion of the Once Daily Human GLP-1 Analog liraglutide in Healthy Male Subjects and its In Vitro Degradation by Dipeptidyl Peptidase IV and Neutral Endopeptidase Drug Metab Dispos. 2010 Aug 13.
An overview of the Liraglutide clinical development program for type 2 diabetes can be reviewed at Liraglutide LEAD Trials Phase 3 Data
Liraglutide has also been studied as an investigational agent for the treatment of obesity in non-diabetic subjects. A range of doses, from 1.2-3.0 mg once daily was administered once daily to obese subjects for 20, weeks, followed by a 84 week open label extension. Control subjects received placebo injections or orlistat 120 mg three times daily. The mean weight loss observed with liraglutide was 4.8, 5,5, 6.3 and 7.2 kg forr doses of 1.2, 1.8, 2.4 and 3.0 mg once daily, respectively. Blood pressure reduction was also observed in liraglutide-treated subjects. Individuals were also advised to follow a calorie reduced diet, and were encouraged to increase physicial activity. All liraglutide-treated patients started at 0.6 mg once daily, and doses were increased during the first 2-4 weeks of the study. Heart rate increased by up to 4 bpm on liraglutide, and nausea and vomiting were the most commonly reported AEs, and patients on orlistat reported more gastrointestinal complaints than those treated with liraglutide. No pancreatitis was observed and no significant changes in calcitonin levels were reported, although the calcitonin data was not provided in the manuscript. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study Lancet. 2009 Nov 7;374(9701):1606-16
Liraglutide has also been evaluated in exploratory studies for the treatment of type 1 diabetes. Both C-peptide(+) and C-peptide(-) patients were studied; All C-peptide(+) patients were treated with liraglutide, whereas 19 C-peptide(-) patients were randomized to either addition of liraglutide or simply continuation on insulin. Patients treated with liraglutide managed to reduce their daily dose of insulin (predominant reduction in rapid acting insulin) and two C-peptide(+) patients discontinued insulin entirely. Liraglutide therapy was associated with a modest but non-significant decrease in A1c and weight loss, and a reduced amount of time spent with a glucose less than 3.9. Four weeks of liraglutide treatment had no effect on stimulated C-peptide levels. Gastrointestinal complaints, predominantly nausea, were very common during the first few days of therapy in patients receiving liraglutide. There was no significant difference in development of exercise-induced hypoglycemia on or off liraglutide. The mean weight loss with liraglutide was ~ 2.3 kg. Four Weeks of Treatment With Liraglutide Reduces Insulin Dose Without Loss of Glycemic Control in Type 1 Diabetic Patients With and Without Residual {beta}-Cell Function Diabetes Care. 2011 May 18.
Liraglutide was compared in a head to head studies with the DPP-4 inhibitor sitagliptin in patients not achieiving adequate glycemic control on metformin. A 26 week open label randomized study of liraglutide 1.2 or 1.8 mg vs sitagliptin 100 mg daily in a study population with a mean baseline HbA1c of ~ 8.5%. Liraglutide was more significantly effective than sitagliptin in regard to absolute reduction of HbA1c (1.2 and 1.5% vs. 0.9%), proportion of subjects getting to goal (55% vs 20%), and weight loss (~3 kg vs 1 kg). This data was disclosed on August 6 2009 at the Novo Nordisk quarterly earnings call and press release.
The long-acting DPP-4-resistant GLP-1R agonist liraglutide was assessed in a randomized study of patients with T2DM for 5 weeks with metformin plus liraglutide, liraglutide alone, metformin alone, or metformin plus glimepiride (open label). The dose of liraglutide was increased weekly and ranged from from 0.5 to 2 mg daily. Liraglutide added to metformin monotherapy was significantly reduced fasting glucose (-3.9 mM -4.9; -2.9) and HbA1c (-0.8% -1.2; -0.4) together with a reduction in body weight (-2.9 kg in the metformin + liraglutide group). See Five Weeks of Treatment with the GLP-1 Analogue Liraglutide Improves Glycaemic Control and Lowers Body weight in Subjects with Type 2 Diabetes. Exp Clin Endocrinol Diabetes. 2006 Sep; 114 (8):417-23
Subsequent studies evaluated the use of liraglutide as monotherapy in patients with type 2 diabetes controlled on diet alone, or following discontinuation of oral agents. Fasting plasma glucose at the time of randomization was 7-13 mM. Three doses of liraglutide were examined; 0.625, 1.25 and 1.9 mg daily. Liraglutide was well tolerated and resulted in placebo-subtracted changes in HbA1c of up to 1.7% after 14 weeks of therapy, with 46% of patients reaching a HbA1c of less than 7% at the highest dose examined. Liraglutide therapy also reduced the proinsulin;insulin ratio, and a mean weight loss of almost 3 kk was observed in patients receiving 1.9 mg daily. See Liraglutide, a long-acting human GLP-1 Analog, given as Monotherapy Significantly Improves Glycemic Control and Lowers Body Weight without Risk of Hypoglycemia in Patients with Type 2 Diabetes Mellitus. Diabetes Care. 2007 2007 Jun;30(6):1608-10.
Th efficacy of liraglutide was assessed in Japanes subjects with type 2 diabetes, mean pre-treatment hbA1c of 8.3%, as monotherapy, in some patients after OAD washout, for 14 weeks. Liraglutide therapy was associated with substantial dose-dependent reductions in HbA1c, fasting and postprandial glucose, and beta cell function. Unexpectedly, no weight loss was observed in this study. See Dose-dependent improvement in glycemia with once-daily liraglutide without hypoglycemia or weight gain: A double-blind, randomized, controlled trial in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2008 May 19. [Epub ahead of print]
Liraglutide has been compared in a head to head study with twice daily exenatide in subjects with type 2 diabetes taking metformin, a sulfonylurea, or both. Liraglutide produced supperior reductions in A1c relative to exenatide (1.1 vs 0.8%), was more potent at reducing fasting glucose, less effective at controlling postprandial glucose, associated with slightly less nausea, and allowed more patients to achieve ADA glycemic targets, with similar reductions in body weight observed for both exenatide and liraglutide (about 3 kg). See the LEAD 6 Study Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009 Jul 4;374(9683):39-47
In the 14 week extension study of LEAD 6, patients previously treated with maximally tolerated stable doses of metformin, sulfonylurea, or both for ≥3 months were randomized to exenatide or liraglutide for 26 weeks, then those receiving twice daily exenatide were switched, in a non-randomized extension study, to once daily liraglutide with dose escalation to 1.8 mg daily, which was complete by the third week of the extension phase. Remarkably, 97% of the original study population completed the extension phase. There was an additional reduction in mean A1c of 0.3% (from 7.2 to 6/9%) in subjects switched to liraglutide , with additional reductions in FPG, body weight and systolic blood pressure. Switching to once-daily liraglutide from twice-daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care. 2010 Jun;33(6):1300-3.
Liraglutide has also been administered in combination with insulin in exploratory clinical investigational programs. The combination of liraglutide and insulin determir, in short term single dose pharmacokinetic studies, and after 3 weeks of liraglutide administration, did not impact the pharmacokinetic profiles exhibited by either liraglutide or detemir when administered alone in subjects with T2DM, as outlined in Co-administration of liraglutide with insulin detemir demonstrates additive pharmacodynamic effects with no pharmacokinetic interaction Diabetes Obes Metab. 2011 Jan;13(1):75-80.
Phase 3 Clinical Trial Data in Press Release form for Liraglutide
Phase 2B Clinical Trial results for Liraglutide were provided by Novo Nordisk in a November 25 2005 Press Release
A clinical trial of single dose subcutaneous of Liraglutide (NN2211) was carried out in healthy male subjects utilizing a randomized double blind design with a dose escalation ranging from 1.25-20 ug/kg. No serious adverse reactions were reported, and all subjects completed the study, however headaches, dizziness, nausea and vomiting were reported more frequently in drug-treated subjects. Nausea and vomiting were more prominent at the higher dosing levels. Only modest glucose lowering effects were seen in the normal subjects, with insulin levels trending lower. Insulin secretion was increased following IVGTT in Liraglutide-treated subjects. See Pharmacokinetics, Pharmacodynamics, Safety, and Tolerability of a Single-Dose of NN2211, a Long-Acting Glucagon-Like Peptide 1 Derivative, in Healthy Male Subjects. Diabetes Care. 2002 Aug;25(8):1398-404 and The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia. 2002 Feb;45(2):195-202
Liraglutide was administered to 11 patients with mild Type 2 diabetes, mean age 59, HbA1c 6.5%, mean duration of diabetes 2.7 years (range 2 months to 12 years). Treatment with oral agents was stopped 2 days before the study period. Liraglutide 10 ug/kg was given by sc pen injection into the abdomen at 23:00. Blood sampling in the fasting and postprandial state was then used to assess the effect of liraglutide, and gastric emptying was assessed by co-administration of 3-OMG with a 2500 kJ meal at 11:30 am the next day.
The key findings from this study include a decrease in both fasting and meal-related glycemic excursion, and an increase in insulin secretory capacity as assessed by HOMA. Pharmacokinetic profiling revealed a drug t1/2 of ~10+ 4 hours. Two patients experienced treatment-associated nausea, one mild, and one moderate, and these patients had the highest peak levels of liraglutide (over 11 nM). These findings illustrate the therapeutic properties of this analog, and highlight some of the challenges for therapeutic delivery of a GLP-1 analog, namely balancing maximum efficacy with tolerability across a broad spectrum of patients. To review the data, see Bedtime Administration of NN2211, a Long-Acting GLP-1 Derivative, Substantially Reduces Fasting and Postprandial Glycemia in Type 2 Diabetes. Diabetes. 2002 Feb;51(2):424-429
Subjects treated with varying doses of once daily liraglutide (193) with a mean initial HbA1c of 7.6% were randomly assigned to fixed-dosage groups ranging from 0.045, 0.225, 0.45, 0.60, or 0.75 mg), placebo, or open-label sulfonylurea (glimepiride, 1-4 mg). In the 0.75-mg liraglutide group, HbA(1c) decreased by 0.75, body weight decreased by 1.2 kg in the 0.45-mg liraglutide group and the proinsulin-to-insulin ratio was also improved (decreased). See Improved Glycemic Control With No Weight Increase in Patients With Type 2 Diabetes After Once-Daily Treatment With the Long-Acting Glucagon-Like Peptide 1 Analog Liraglutide (NN2211): A 12-week, double-blind, randomized, controlled trial. Diabetes Care. 2004 Jun;27(6):1335-42
Liraglutide also improves selected parameters of β-cell function in human subjects with type 2 diabetes. A 14 week study of patients with T2DM treated with different doses of liraglutide revealed improved glucose-and arginine-stimulated insulin secretion, as outlined in Liraglutide, a once-daily human GLP-1 analogue, improves pancreatic β-cell function and arginine-stimulated insulin secretion during hyperglycaemia in patients with Type 2 diabetes mellitus. Diabet Med. 2008 Jan 14; [Epub ahead of print]
The mechanisms whereby Liraglutide regulates islet cell function was examined in a short term study of patients with T2DM One week's treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes. 2004 May;53(5): 1187-94
An 8 week study of liraglutide in 33 obese subjects with Type 2 diabetes with a mean entry HbA1c examined glycemic control, body weight and energy expenditure in subjects treated with a single daily dose of 0.6 mg per day. Liraglutide reduce HbA1c (0.33%), decreased plasma glucose, prevented weight gain, with no effect on energy expenditure. See The Effect of Liraglutide, a Long-Acting Glucagon-Like Peptide 1 Derivative, on Glycemic Control, Body Composition, and 24-h Energy Expenditure in Patients With Type 2 Diabetes Diabetes Care. 2004 Aug;27(8):1915-21.
A 12 week study of Liraglutide in assessed the efficacy of once daily liraglutide compared to metformin in type 2 diabetes. Liraglutide produced comparable HbA1c reduction with prevention of weight gain, as described in Effects of liraglutide (NN2211), a long-acting GLP-1 analogue, on glycaemic control and bodyweight in subjects with Type 2 diabetes. Diabet Med. 2005 Aug;22(8):1016-23
Liraglutide - Preclinical Data
Liraglutide has been studied in a number of different of preclinical models. Liraglutide at a comparatively large doses of 200 ug/kg twice daily for 10 days inhibited food intake and decreased body weight in MSG-treated obese rats. A 7-day Liraglutide treatment of normal rats produced a decrease in energy expenditure, however normalizing the effect for the degree of weight loss revealed no change in EE per weight unit of lean body mass. See Systemic administration of the long-acting GLP-1 derivative NN2211 induces lasting and reversible weight loss in both normal and obese rats. Diabetes. 2001 Nov;50(11):2530-9
Similarly, administration of liraglutide to db/db mice twice daily for several days, or for 2 weeks, reduced weight gain, increased the expression of genes important for cell cycle regulation and apoptosis, enhanced b-cell proliferation and b-cell mass, reduced triacylglycerol accumulation in islets, decreased apoptosis, and improved GSIS. Whether these effects were predominantly related to improvements in metabolic control remains unclear, as only some, but not all of the effects persistent in non-diabetic control mice, which also exhibited reduced weight gain The human glucagon-like peptide-1 analogue liraglutide preserves pancreatic beta cells via regulation of cell kinetics and suppression of oxidative and endoplasmic reticulum stress in a mouse model of diabetes Diabetologia. 2011 May;54(5):1098-108
Anti-inflammatory actions of liraglutide: Liraglutide action has been studied in different cell models, including human vascular endothelial (HUVEC) cells. Liraglutide increase NO production, and rapidly enhanced eNOS, AMPK, and ACC phosphorylation independent of cyclic AMP activation. Liraglutide attenuated the effects of TNFalpa on eNOS expression in an AMPK-dependent manner. Liraglutide also reduced the degradation of IkB-a and inhibited high glucose-induced NF-kB activation in HUVEC cells. These findings demonstrate a range of anti-inflammatory actions of liraglutide, acting in part through AMPK-dependent pathways, in human endothelia cells A glucagon-like peptide-1 (GLP-1) analogue, liraglutide, upregulates nitric oxide production and exerts anti-inflammatory action in endothelial cells Diabetologia. 2010 Jul 1. [Epub ahead of print]
The properties of Liraglutide have also been examined in mice and pigs. Liraglutide dose-dependently reduced blood glucose in ob/ob mice, with evidence for antihyperglycemic activity still evident 24 h after Liraglutide administration. Although an initial reduction in food intake was observed, body weight was not affected during the 2 week study period. Consistent with the effects of other GLP-1R agonists, Liraglutide increased β cell proliferation and β cell mass. See The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice. Am J Physiol Endocrinol Metab. 2002 Oct;283(4):E745-52
The acute and chronic effects of Liraglutide have been examined in minipigs. Liraglutide acutely increased insulin and inhibited glucagon secretion, and decreased gastric emptying, similar to findings with other GLP-1R agonists. Liraglutide also acutely increased glucose utilization during a hyperglycaemic glucose clamp. See NN2211: a long-acting glucagon-like peptide-1 derivative with anti-diabetic effects in glucose-intolerant pigs. Eur J Pharmacol. 2002 Sep 13;451(2):217-25
A head to head study of the DPP-4 inhibitor Vildagliptin vs. Vildagliptin, however there were no major differences in glucose or HbA1c, whereas plasma insulin levels were significantly higher in rats treated with Vildagliptin. Both Vildagliptin and β-cell mass compared to vehicle-treated candy-fed rats. Hence, both drugs appear to be reasonably potent controlling blood glucose, whereas GLP-1R agonists may be associated with reduced food intake and weight loss. See Liraglutide, a Long-Acting Glucagon-Like Peptide-1 Analog, Reduces Body Weight and Food Intake in Obese Candy-Fed Rats, Whereas a Dipeptidyl Peptidase-IV Inhibitor, Vildagliptin, Does Not. Diabetes. 2007 Jan;56(1):8-15
