Finan et al studied the co-administration of equimolar [] of a GLP-1R and a GIPR agonist, revealing greater reduction in food intake, body weight, and fat mass in mice with HFD-fed diet-induced obesity relative to administration of the single agents alone. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans Sci Transl Med. 2013 Oct 30;5(209):209ra151 findings replicated in additiional preclincial studies examining combined activation of both the GLP-1R and GIPR as reviewed here Glucagon-like peptide-1 receptor co-agonists for treating metabolic disease Mol Metab. 2021 Apr;46:101090. doi: 10.1016/j.molmet.2020.101090.
Unimolecular GLP-1:GIP co-agonists
A 40 amino acid single peptide chimera on a glucagon backbone xhibited balanced activity at the GLP-1 and GIP receptors, minimal GCGR activity with prolonged action in vivo Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans Sci Transl Med. 2013 Oct 30;5(209):209ra151. This co-agonist dose-dependently reduced body weight, and adipose tissue mass, with greater efficacy vs. equimolar doses of exendin-4 and liraglutide.
Single dose administration of the PEGylatedGLP-1:GIP co-agonist in healthy humans increased insulin secretion and lowered blood glucose levels in the context of an associated glucose infusion. Analysis of 53 subjects with T2D treated with once-weekly co-agonist revealed dose-dependent reductions in HbA1c ( -0.53% to -1.-11%)..
RG7697/NNC0090-2746
The acylated version of the GLP-1:GIP co-agonist originally described by Finan and colleagues (described above) is represented by the designations RG7697 and NNC0090-2746 Pharmacodynamics, pharmacokinetics, safety and tolerability of the novel dual glucose-dependent insulinotropic polypeptide/glucagon-like peptide-1 agonist RG7697 after single subcutaneous administration in healthy subjects Diabetes Obes Metab. 2017 Oct;19(10):1446-1453. Single dose RG7697/NNC0090-2746 was evaluated in 51 healthy volunteers
Pharmacodynamics, pharmacokinetics, safety and tolerability of the novel dual glucose-dependent insulinotropic polypeptide/glucagon-like peptide-1 agonist RG7697 after single subcutaneous administration in healthy subjects Diabetes Obes Metab. 2017 Oct;19(10):1446-1453 Doses of G7697/NNC0090-2746≥ 1.8 mg, reduced glucose and insulin levels during a meal tolerance test. RG7697/NNC0090-2746 aand once daily administration for 2 weeks reduced HbA1c by -0.67% and body weight (-3.0 kg). RG7697/NNC0090-2746 1.8mg daily was evaluated in 108 people with T2D inadequately with a comparator of daily njections of liraglutide. RG7697/NNC0090-2746 reduced HbA1c levels and body weight with reductions similar to those achieved with liraglutide The Sustained Effects of a Dual GIP/GLP-1 Receptor Agonist, NNC0090-2746, in Patients with Type 2 Diabetes Cell Met 2017 Aug 1;26(2):343-352.e2.
Tirzepatide (LY3298176)
Although several GIP-GLP-1 co-agonists initially failed to impress in clinical testing, more recent data for trizepatide (originally known as LY3298176) demonstrated compelling reduction of blood glucose and body weight in both preclinical LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept Mol Metab. 2018 Dec;18:3-14. doi: 10.1016/j.molmet.2018.09.009 and clinical studies. Tirzepatide (LY3298176) is a 39 amino acid peptide based on a GIP sequence modified to include substitution of the second amino acid with aminoisobutyric acid (DPP-4 resistance) and containing a C20 unsaturated di-acid acyl chain, to enable non-covalent albumin binding and once-weekly subcutaneous dosing LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept Mol Metab. 2018 Dec;18:3-14. doi: 10.1016/j.molmet.2018.09.009 Tirzepatide exhibits comparable binding affinity and potency at the human GIPR relative to native GIP yet binds with 5-fold less affinity and is 13-fold less potent at the human GLP-1R, compared to native GLP-1 thus exhibiting bias for the GIPR. Several of the key dose rangong studies and clinical trials are highlighted here Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial Lancet. 2018 Nov 17;392(10160):2180-2193. An overview of the Phase 3 Tirzepatide SURPASS program for Type 2 diabetes was presented by Eli Lilly on Nov 20 2020 and the SURPASS trials have been published highlighting the efficacy of Tirzepatide in people with T2D. The SURPASS phase 3 clinical trial program assessed the efficacy of Tirzepatide in people with type 2 diabetes. The results are summarized below, for HbA1c and weight loss
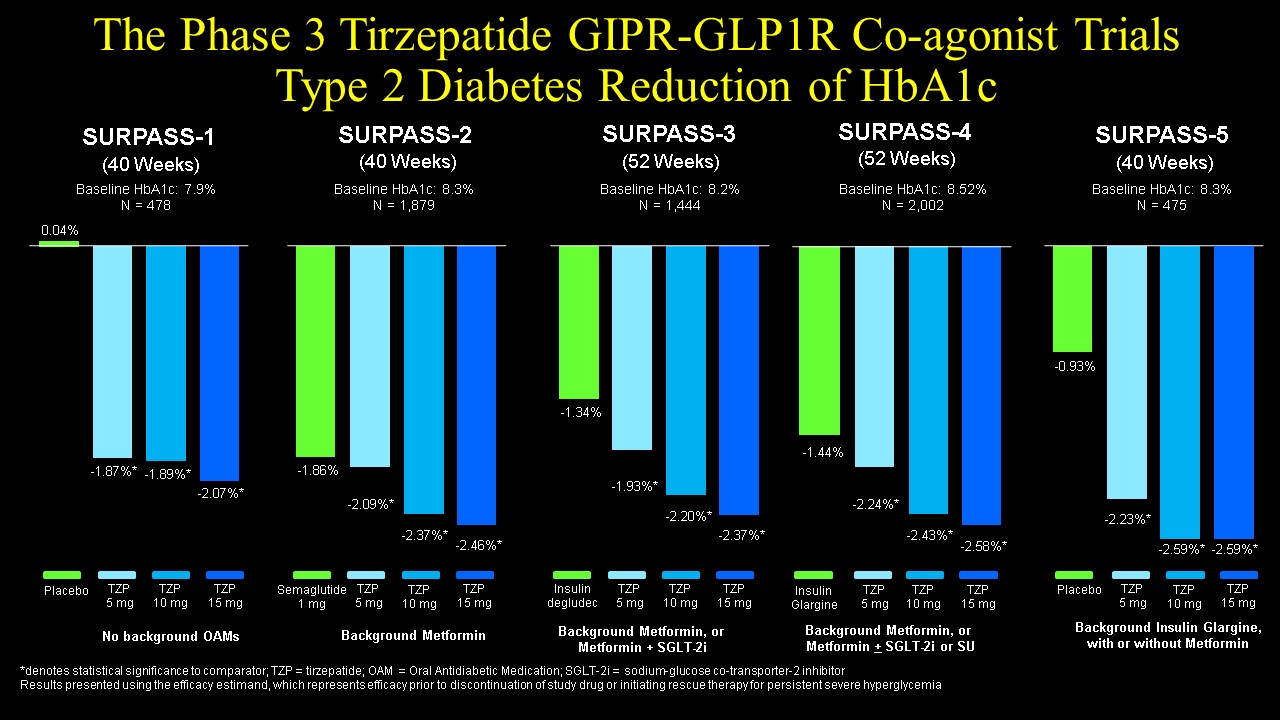
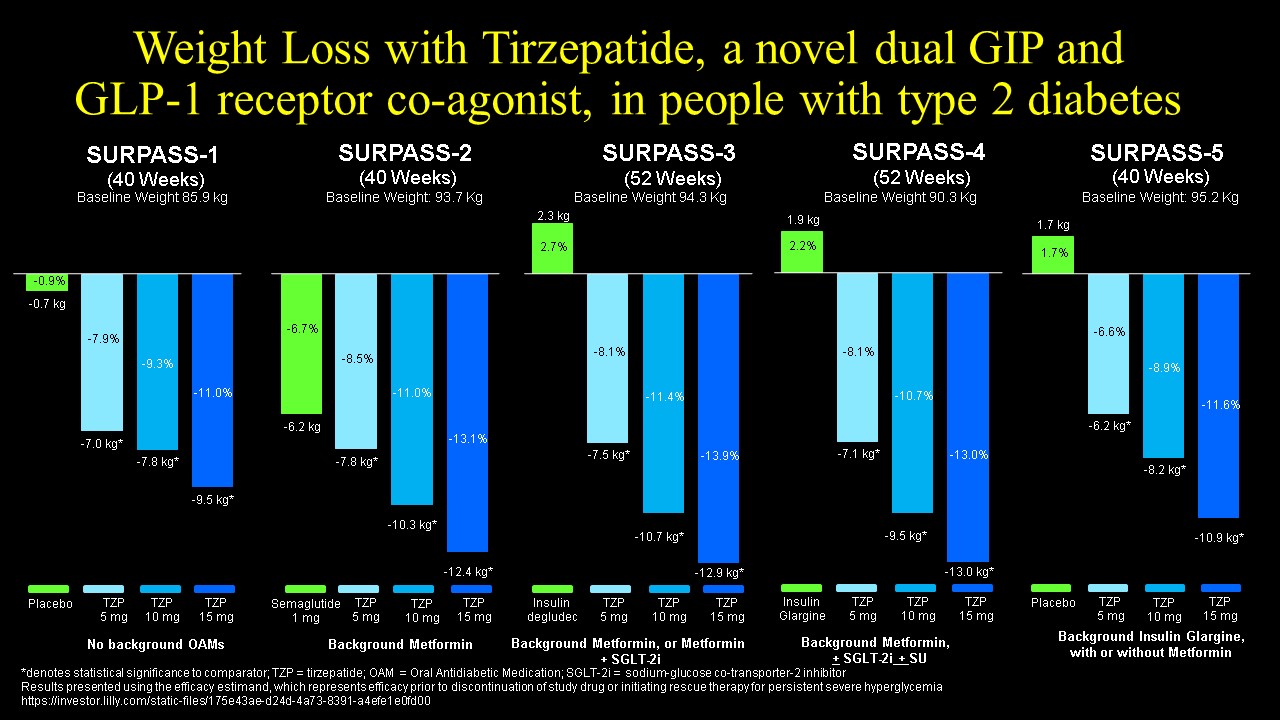
The cardiovascular safety of once weekly tirzepatide is currently being compared to that of dulaglutide in the SURPASS cardiovascular outcome trial. This study has a primary endpoint of 3-point Major Adverse Cardiovascular Events (MACE; myocardial infarction, stroke, and cardiovascular death), is expected to randomize 12,500 subjects with T2D, and conclude in the third quarter of 2024.
